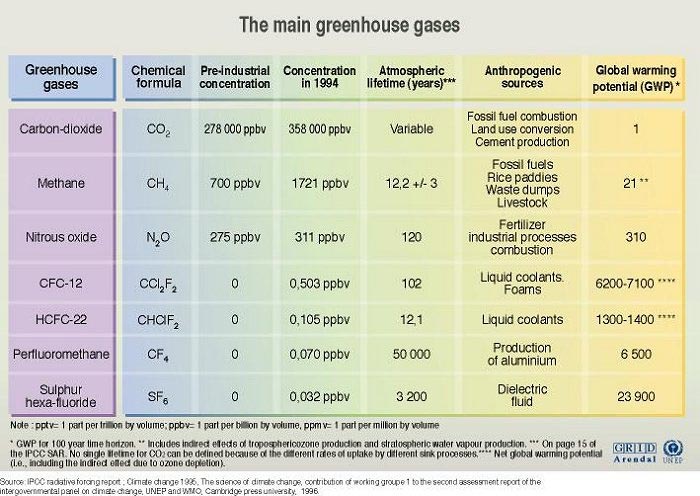
How much do you know about the perils of a carbon and its four hydrogen friends—otherwise known as methane? The environmental media is usually inundated with a list of carbon related discussions that is inexhaustible. It is fair to say that the psyche of the greeniac has long been dominated by the perils of carbon dioxide and its nefarious reputation as a greenhouse gas that is destined to greenhouse gas in the atmosphere is in fact estimated to cause 20 times more warming than carbon dioxide on a global scale 1 and yet we barely hear about it on popular media outlets. The total amount of methane has more than doubled over the last 200 years to nearly 1700 ppb (part per billion) from 800 ppb,2 and human activity will likely keep these abnormally high levels of methane in circulation.
 i
i
Methane and Climate Change
Combining increasing global atmospheric levels of methane with a much higher heat trapping ability, the atmospheric circulation of methane is a recipe for disaster as an accelerant for climate change . But how can a tetrahedral, highly reduced gas be so efficient at absorbing heat? The answer comes from the chemistry of bond formation in chemical compounds, which exhibit very unique properties. The chemical formula of methane is CH4, therefore consisting of four C-H bonds in the molecule. The most well understood mechanism by which molecules absorb energy is in fact through their bonds, which is an arrangement of shared electrons that exhibit unique quantum mechanical formula. Electrons in bonds can be excited to higher energy states through energy absorbance, and these excitations will always occur at discreet energy levels. As a result, a C-H bond will always absorb a similar amount of energy even in a different molecule because of the electronic nature of the bond. As a molecule having four bonds, as opposed to two bonds in carbon dioxide (CO2), a basic framework of thinking about methane is by imagining the molecule having four bonds to absorb energy. Most importantly, however, the C-H bond in methane absorbs energy in the infrared range 3 which corresponds to thermal energies from the sun. The net result is that methane has a much higher thermal energy absorbance capacity than carbon dioxide, and currently accounts for 9% of all greenhouse gas emissions in the U.S.4

Most of the methane being emitted in our atmosphere is the result of human activity. For example, while many tout natural gas as being a clean energy, a good deal of methane is released into the atmosphere throughout the extraction to market processes of natural gas.5 Many underground oil reserves have pockets of methane gas that leak out during drilling activities, and this is typically burned off to power the plant and remove methane from the reservoir.6
Interestingly, the second largest source of atmospheric methane is derived from anaerobic organic degradation by bacteria.7 Non-oxygen using bacteria are well known for having evolved diverse mechanisms of metabolism for energy and the breakdown of organic matter for carbon—one such mechanism produces methane gas. This reaction is in fact harnessed in many wastewater plants to degrade organic pollutants in water.8 In fact, there have been many studies into the practical application of methane production through extraction from water plants and treatment of animal manure 9 to produce methane for low emissions energy production—think trapping cow farts ☺.

Since we are on the topic of manure, you might ask why manure could possibly produce methane? While many anaerobic organisms may be found in natural environments such as swamps, these bacteria are abundant in the gut of livestock. As a result, the supposed “natural” source of methane from anaerobic bacteria is actually directly from the human activity of livestock rearing. To put it simply, a belching and farting cow has become the bane of our greenhouse gas problem. A growing demand for animal protein is inadvertently creating biological factories of methane producers, responsible for 14% of the greenhouse gas emissions on Earth.10 There are billions of cows and other grazing animals around the world that are gassing up the planet with ammonia (NH3 pollution as well. One of the reasons cows and other grazers are so adept at producing methane is their unique anatomy of having four stomachs that are laden with anaerobic bacteria.
There is some good news—methane emission rates have actually decreased by around 11% from 1990 to 2012.11 The bad news is that there is still more than 500 million metric tons of CO2 equivalent methane being released into the atmosphere.12 Interestingly, the distribution of methane emission sources have changed over this time, with more emissions attributable to agricultural activities and less to energy exploration. There is, however a potentially new source of methane buried as an icy pressurized solid known as methane hydrates.13 Buried under permafrost and interlocked in a cage of water molecules, the United States Geological Survey (USGS) estimated in 2008 that as much as 157.8 trillion cubic feet of methane to be buried underground waiting to be released under normal atmospheric conditions.14 Ironically, the most well documented method of extracting methane from hydrates is by injecting reservoirs with global warming essentially melting frozen hydrates located close to the earth’s surface, some scientists have speculated these melting events as catastrophic time bombs having serious environmental as well as economic effects.15
https://web.archive.org/web/20160404083020if_/http://www.youtube.com/embed/NVpQnpWS2wU iii
Controlling Methane Going Forward
While methane emissions may be increasing rampantly, there are numerous advocacy oriented measures we can take to curb methane emissions. Promoting a reduction in fossil fuel usage would inevitably reduce methane emissions by reducing leakage events from fossil fuel exploration activities. There must also be a strong foundation in establishing sustainable agricultural practices. Manure management strategies can be implemented to harvest methane containing natural gas to efficiently power the farm 16 along with more research into feeding regimes that reduce the production of methane by livestock in the first place. Additionally, more advanced infrastructure in landfill sites to isolate and harvest methane from decomposing organic matter as opposed to allowing for the gas to escape. Most importantly, however, we must all be actively involved in collective outreach and education for methane awareness and its devastating potential in accelerating climate change.