We hear about the “ozone hole” regularly in the news, but what exactly does this mean? Let’s take a look at this environmental and human health problem of a depleting ozone layer. Ozone —the triatomic natural wonder of chemistry that unusually pairs three oxygen atoms—forms nature’s contiguous sun screen against harmful ultraviolet (UV) radiation that emanates from the sun. The functional role of ozone as a UV protectant is underscored by the evolutionary roots of terrestrial creatures, and thus human beings. If it wasn’t for this miraculous quirk of chemistry that allows for oxygen to pair triatomically in the upper atmosphere instead of its most stable diatomic state, UV radiation from the sun would have most certainly confined early life to the deepest corners of the oceans and seas far from the rays of the sun. The planet and its inhabitants’ survival depend on the natural balance of Ozone in the upper atmosphere. Therefore it is of utmost importance to monitor the condition of the Ozone layer, especially with the dramatic increase of global industrialization that has introduced various chemical agents into our atmosphere.
Ozone Layer Basics

The Ozone layer, which makes up a gaseous film of oxygen (O3) surrounding the earth, is located high up in the stratosphere at altitudes a little beyond where commercial aircraft typically fly. Most of our planet’s ozone is concentrated at around 15 -30 kilometers1 above the earth’s surface, but the use of the word concentrated is rather deceptive. Out of perhaps 10 million air particles, 2 million particles exist in the form of normal oxygen gas (O2) while only 3—that’s right, three—particles are ozone molecules (O3).2 A chemical equilibrium that strongly favors oxygen gas (O2) dynamically maintains low concentrations of ozone in the atmosphere, which would seem almost disastrous. This extremely small amount of blue and odorous gas, however, is responsible for absorbing harmful UV radiation, particularly shorter wavelength UVB radiation.
There are in fact many kinds of UV radiation, distinguished by a wave property known as the wavelength that describes the nature of a wave through the length of its cycle. Without delving too much into the physics of waves, the general rule of thumb is that a shorter wavelength wave will carry much more energy than a long wavelength wave. UVB radiation typically falls into a wavelength range of 290 – 320 nanometers,3 while UVA falls in a range of 320 – 400 nanometers. As a result, UVB is often implicated in skin cancers, cataracts and crop damage.4 Fortunately, the Ozone layer does a commendable job absorbing UVB radiation but falls short of absorbing a significant amount of UVA radiation. While newer research does seem to indicate that prolonged UVA exposure may cause skin damage,5 the effects are not nearly as harmful as UVB and therefore the ozone layer still earns a high score and pat on the back from the scientific world.
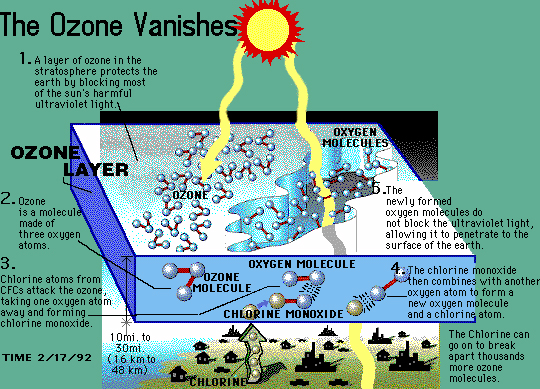 6
6
The Traditional Ozone Hole
As much as a simplified image of a layer of floating gas in the stratosphere serves to introduce the general concept of the ozone layer, the layer is in fact a fairly complex and dynamic shell whose dimensions tend to change seasonally as part of numerous natural cycles on Earth. The ozone layer does not evenly blanket the earth at all times of year, and counterintuitively is lower in concentrations towards the tropics but higher in concentration towards the earth’s poles due to air circulation patterns that redistribute ozone in the earth’s atmosphere.7 Therefore, there is less ozone in the tropics to address the higher intensities of UV radiation that is present near the equator. While the concentrations of ozone are higher in the earth’s poles, they also vary considerably by season in contrast to tropics ozone levels.8
During the northern spring (March – April), the North Pole has been monitored to have the highest concentration of ozone, while the spring season in the South Pole (September – October) has been observed to have the lowest concentrations of Ozone recorded in the atmosphere. The concentrations during the spring season, are in fact so low in the Antarctic that an ozone hole forms as part of a natural earth cycle. Hence the foundation for the previously held notion of the Ozone hole being a natural, i.e. not man-made, phenomenon. As long as such a hole was localized to the Antarctic and for the period of a few months, there was little reason for humans or animals to fear. Unfortunately the size and temporal presence of this hole has risen alarmingly, with some estimates of the Antarctic ozone hole reaching 27 million square kilometers, and much of this increase can be linked to human activity.9 One of the major consequences of this depleted ozone layer in the Arctic has been rising sea levels .
https://web.archive.org/web/20160404101517if_/http://www.youtube.com/embed/k2kpz_8ntJY 10
Ozone Depletion
The dramatic increase in the size of the ozone hole had alarmed atmospheric scientists for years, and the culprit of anthropogenic ozone depletion was discovered as far back as the early 1970’s.11 Chlorofluorocarbons (CFCs) are one of the most publicized names that is thrown around in discussions of ozone depletion, but there is in fact a vast array of chemical agents linked to ozone depletion in the upper stratosphere. Other examples of ozone depleting chemicals include industrial solvents such as methyl chloroform and carbon tetrachloride, as well as fire retardants such as halons, and soil fumigants such as methyl bromide. The commonality between all agents mentioned so far are their halogen content—the presence of halogen atoms that form a group of elements in the periodic table. Some common halogens include chlorine, bromine, and fluorine, and their interaction with ozone molecules through some interesting chemistry explains the depletion of ozone molecules from the upper atmosphere.
Halogen containing compounds such as chlorofluorocarbons are able to partially disassociate in the upper atmosphere when exposed to high energy UV light. During this process, a free chlorine atom can freely react with up to 100,000 ozone molecules through a sustained chain reaction that depletes ozone at an alarming rate.12 There are a wide variety of chemical agents that contain halogens that are commercially used, so it may seem puzzling that CFCs are a particular cause for concern. The stability of the compound, a desired property that had made CFCs a commercial success, ensures that CFCs only break down in the upper levels of the atmosphere that unfortunately contain ozone molecules.
There are, however, a variety of atmospheric phenomena that exacerbate ozone depletion in the presence of CFCs. Volcanic eruptions may increase the level of aerosols in the atmosphere that contribute to the reactivity of free chlorine, instrumental in ozone damage. Polar stratospheric clouds13 formed under extremely cold conditions in the Antarctic provide a particulate surface that may activate chlorine in the atmosphere. This phenomena is particularly limited to the Antarctic where polar vortices isolate cold air currents to the South Pole and significantly contribute to seasonal variations in ozone levels. This effect is further exacerbated by the collection of ozone depleting substances towards the South Pole through wind currents that collect substances such as CFC’s in the Antarctic. Therefore, the use of CFC’s anywhere in the world could potentially widen the ozone hole in the Antarctic, and this revelation certainly underscores the need for sustainable action and scrutiny against ozone depletion.
The Ozone Holes
Unfortunately, the Antarctic ozone is just the tip of the iceberg. While ozone depleting substances are acting to widen the Antarctic ozone hole, the greater concern for the issue of the ozone layer is plural. There are in fact numerous ozone “holes” in areas of Europe, Australia, as well as North America. Australia has one of the highest rates of skin cancer in the world,14 and much of this can be attributed to high UV exposure linked to the Antarctic ozone hole that has widened enough to affect the Australian continent. Data compiled In Switzerland identified a progressive drop in atmospheric ozone by 2.1% between 1973 and 2001.15
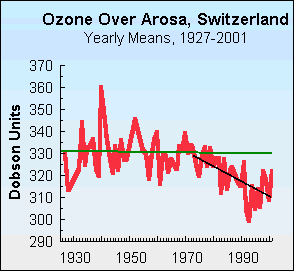 16
16
Similarly in Los Angeles, Miami, and Seattle ozone levels had been monitored as dropping from the years 1979 to 1994.17 While the data seems quite old, there are very few recent ozone monitoring studies in the North America region and current statistics suggest an average loss of 5- 10% of the ozone layer in the United States.18 While the percentages may appear deceptively small, the ozone layer itself is relatively thin in composition, and therefore even small changes to the layer could produce macroscopically deleterious effects. Ozone depletion is further complicated by altitude dependency, such that higher altitude cities, such as Seattle, will be more affected than lower altitude cities such as Miami. The urgency of limiting ozone depletion in the Antarctic and globally cannot be understated, especially with the alarming prevalence of melanoma skin cancer in the Australian continent. The metastatic and debilitating effects of skin cancer combined with the potentially devastating ecological damage to numerous plant and animal species underscore the need for sustainable action to maintain the good health of the ozone layer.
Sustainable Global Action
Modern ecological and environmental studies are increasingly elucidating the harmful macroscopic effects that industrialization has brought forth to the delicate abode of Mother Nature. Of the varying issues that are increasingly being studied, those that affect the fundamentals of our survival are particularly of high priority to our society its existence. Time and again, a pervasive inability to perceive the imminent dangers of an endangered environment and the continual destruction of our environment have revealed the difficulties in enacting sustainable global change. The global response to ozone depletion, however, is perhaps one of the few instances in which transnational action has yielded widespread success. The world’s response to ozone depletion came in the form of the Montreal Protocol of 1987 that has been ratified by all UN member nations and hopes to phase out the production of ozone depleting substances.19 The treaty was further modified in 1992 in response to newer scientific studies to include new expedited targets such as the phase-out of halons by the beginning of 1994, as well as that of CFCs in the beginning of 1996.20 The United States is currently on schedule to phase out first generation and second generation ozone depleting substances and in fact is ahead of schedule for the phasing out of hydrochloroflouro carbons, a former substitute for CFCs.21
Parting Words on Ozone Depletion
While the global depletion of ozone has slowly come to a stop, the recovery of lost ozone levels appears to be slow and painful with a projected recovery to pre-1980 levels not expected before 2060 at best.22 Although the damage to the ozone is still pronounced, the level of international cooperation for this environmental issue is almost unprecedented. While the ozone hole does stand as a physical threat to all natural life on our planet, it is also symbolic of the unknown dangers of environmental destruction that may not be as apparent, as well as the limitless potential of international cooperation to preserve our environment.