Our society is intricately bound to fertilizer. 200 years ago, prominent English economist Thomas Malthus predicted an imminent collapse of populations around the world centered on an idea that food production could never meet the growth rate of an exponentially multiplying population.1 Indeed, as animals we would certainly be limited to this fate if it wasn’t for human ingenuity and creativity to discover a means of subsistence, specifically by finding ways of making the plant more efficient. One of the first ways of ever achieving this feat was through the use of fertilizers, a practice dating back to over 8000 years ago with the use of the world’s first fertilizer—manure. It is estimated that 30 to 50% of the crop yield around the world can be attributed to fertilization.2 That equates to around 170 million tons of fertilizer currently being used worldwide.3 Therefore, fertilizers are intricately connected to the way we eat and thrive on a daily basis.
Fertilizer Production

A fertilizer in the most general sense is a chemical additive or growth supplement that enables a plant to proliferate and achieve efficient growth through the abundance of mineral resources. As autotrophs that are able to produce their own source of carbon and energy through photosynthesis, the major limitation of plants is not necessarily its bulk food supply—i.e. sugars derived from photosynthesis. With the mass abundance of nitrogen ![]() , phosphorus and potassium.4 These three minerals are even central to human development, as any living system is in need of these key minerals to produce proteins, nucleic acids and power the numerous ion pumps 5 needed to power critical physiological processes in living organisms. For human beings the consumption of various types of foods, which are normally fortified by these essential minerals in the manufacturing process, more than satisfies our own mineral requirement. Plants, on the other hand, are dependent on the soil content of minerals that can be artificially supplemented with fertilizer.
, phosphorus and potassium.4 These three minerals are even central to human development, as any living system is in need of these key minerals to produce proteins, nucleic acids and power the numerous ion pumps 5 needed to power critical physiological processes in living organisms. For human beings the consumption of various types of foods, which are normally fortified by these essential minerals in the manufacturing process, more than satisfies our own mineral requirement. Plants, on the other hand, are dependent on the soil content of minerals that can be artificially supplemented with fertilizer.
https://web.archive.org/web/20160404103202if_/http://www.youtube.com/embed/mZ5OohqO9b4 i
The dynamics of fertilizer use are perhaps best understood by chronologically following their application in history. Manure, even today, is one of the most widely used organic fertilizers around the world.6 Although becoming rather uncommon in the developed world, the developing world is fueled by this free and highly effective form of fertilization that dates back thousands of years. Surprisingly, there is a vast selection of livestock that is capable of producing effective fertilizer.7 While many livestock consume hundreds of pounds of food over a relatively short period of time, their innate biological inefficiency in digestion means that as much as 75-90% of what they consume comes out the other end.8 For the prospect of fertilization, this is fantastic. As a result, animal waste tends to be loaded with organic waste containing broken down products rich in the three essential minerals. Additionally, since manure is biologically active, its slow breakdown by bacteria allows it to be a source of minerals for an extended period of time. The essential mineral content of fertilizers are usually reported as a relative ratio on the back of packages of nitrogen: phosphorus : potassium (NPK rating).9 Dairy cow manure is the most widely used organic fertilizer with a modest NPK rating of 25%:15%:25%.10 While this may seem low compared to other manure types, cow manure is the safest to use as it contains very low mineral content, sometimes referred to as hot manure because it can kill plants.
Synthetic Fertilizers
Most fertilizers commonly used on the American landscape, however, are synthetic derivatives created from industrial manufacturing processes.11 Available in numerous formulations that are distributed across soils, synthetic fertilizers usually consist of mineral salts that are water soluble and easy absorbed by the plant roots. Some examples may include sodium nitrates, ammonium sulfates, and chemical amides.12 As synthetic agents, these fertilizers usually consist of a very high concentration of the three essential minerals and are typically used in large scale agriculture as a soil additive as well as in home gardening. Synthetic fertilizers can be purchased in varying degrees of mineral composition depending on the type of crop being fertilized. Alfalfa, for example, requires far more nitrogen in comparison to other high yield crops and would be best fertilized by a fertilizer abundant in nitrogen such as an ammonium salt.13 Crops that are in greater need of phosphorus such as wheat 14 would benefit more from super phosphates,15 rock phosphates, or even bone meal—a fertilizer derived from slaughter house waste products.16
Many synthetic fertilizers can be purchased in a variety of formulations based on the intended use. Many synthetics may come in the form of pellets or premixed soils infused with fertilizer. One form known as liquid fertilizers can be used to deploy an adequate amount of fast absorbing fertilizer to either home lawns or crop fields.17 Unfortunately, a drawback of this formulation is the short shelf life of this form to remain active in the soil. To address this drawback, slow release fertilizers 18 have also been developed that are capable of lasting many long months through slow decomposition reactions.
 ii
ii
Fertilizer’s Negative Effects
Most large scale agricultural operations rely on the application of large quantities of fertilizer using cultivators equipped with spraying apparatus. In fact, the mechanization and consolidation of agricultural practices have created a mass dependency on fertilization without the use of sustainable practices that may curb excess. In the United States alone, it is estimated that 41% of pollution of lakes and rivers ![]() around the world.
around the world.
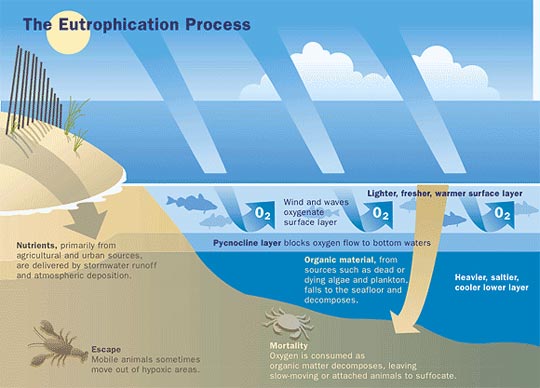
The water soluble nature of fertilizers enable the delivery of macronutrients to roots practically feasible, but this same property inadvertently makes them a primary candidate for agricultural runoff. During seasonal rains or times of irrigation, excess nitrates, phosphates and potassium wash out from soils into lakes and rivers where they wreak havoc by promoting a phenomenon known as eutrophication ![]() .21 Defined as the enrichment of surface waters with plant nutrients, the sudden availability of micronutrients in water bodies promotes the mass proliferation of algae, phytoplankton and macrophytes. Unchecked growth of these organisms starves marine environments of oxygen and nutrients, while potentially releasing toxins into the water resulting in mass fish die outs.22
.21 Defined as the enrichment of surface waters with plant nutrients, the sudden availability of micronutrients in water bodies promotes the mass proliferation of algae, phytoplankton and macrophytes. Unchecked growth of these organisms starves marine environments of oxygen and nutrients, while potentially releasing toxins into the water resulting in mass fish die outs.22
The EPA estimates that nearly 35,000 miles of river across 22 States to be polluted by agricultural waste such as fertilizer,23 and the extent of the pollution is sure to increase without the appropriate interventions against excessive fertilizer usage. While the downstream impacts of fertilizer can devastate aquatic ecosystems, there is also a large upstream consequence of synthetic fertilizer usage. Creating synthetic fertilizer is very energy intensive process, driven by high pressure and temperature reactions—for example, chambers at 200 atm of pressure. It is estimated that 2.5 gallons of fuel are utilized to create a mere 40 pounds of fertilizer,24 which results in the loss of 23.6 billion gallons of fuel annually just from fertilizer the Haber-Bosch 25 process for creating ammonia for nitrogen supplementation run in 400’C usage. Therefore, the environmental impact of fertilizers extends far beyond fossil fuel ![]() consumption.
consumption.
 iii
iii
Fertilizers have undoubtedly revolutionized the world of agriculture by defying the imminent collapse of human populations and creating an abundant supply of crop to feed billions around the globe. The unchecked usage of fertilizer through unsustainable practices, however, represents a grave environmental threat that must be addressed immediately through education, outreach and innovations that promote sustainable agricultural practices.